For the time being, many of the issues in the theory of quantum physics remain little-studied and give no sufficiently complete and logical explanation for the results obtained in practice:
— inconsistency between dependencies on temperature, frequency and wave-length;
— lack of interrelation between the atoms of hydrogen and helium, and correspondingly, between these atoms and atoms of other chemical elements from the periodic table;
— presence of rather complicated and intricate approach to the matter of superfluidity (particularly that of helium ІІ).
The above-mentioned and many other matters, which remain theoretically unexplained today, push the scientists towards seeking for the new approaches, which would ensure complete theoretical explanation for the results obtained in practice.
One of such approaches, which (from the author’s point of view) is totally consistent with the fundamental theoretical conclusions of the founders of quantum physics, is to be presented here.
The following constants (const) are the result of the work done:
«Ш» = 2.32549 • 10-4 (eV/degree) is the universal constant of variation of the potential (electronvolt) with the temperature changing by one degree (Celsius or Kelvin);
«М» = 562.311 • 108 (Hz/degree) is the universal constant of variation of the frequency (hertz) with the temperature changing by one degree (Celsius or Kelvin);
The «2n2» constant is known from the studies of the atom presented in the course of physics. This constant allows determination of the maximum possible number of electrons on the atom n-th shell. Here, the following questions arise: is it the actual number of electrons or is it the number of quantum states of an electron (groups of electrons)? As we know, the number of electrons is different in the atoms of different elements and depends on the charge of the atomic nucleus. Thus, the atom of hydrogen has one electron, the atom of helium has two electrons, the atom of lithium has three electrons, and so on, i.e. the atom of each element has its own group of electrons. The question is whether they are quantized in groups or independently, including quantization within their groups?
In the author’s opinion (confirmed with the following calculations), quantization of electrons takes place in groups, each of which has z electrons, such group having 2n2/z quantum states on the n-th shell in an atom. This rule covers all the atoms (both with the nucleus charge z equal to 1, 2, 3 …, and those with the charge equal to 80, 92, 100, etc.) This is the first of the conclusions in the above arguments laid in the basis of the suggested new method.
Another conclusion should be the recognition of existence in real conditions of conversion of a material particle (an electron) into an electromagnetic pulse and vice versa: the electromagnetic pulse may be converted into electrons by means of «twisting» pulse energy in torsion fields. Knowing these conditions, it is possible to break intermolecular bonds in the exothermal mode, when the process of molecular decomposition takes place not only without extra energy input, but also with the release of large amounts of internal energy of bonds. These are the above-mentioned conversions from the material state into the energetic one, which determine the properties of helium ІІ. It is known that under certain conditions helium II becomes superfluid and acquires extremely high thermal conductivity.
Similar approaches related to the conversion of one type of energy into another also refer to the atomic nucleus -nucleons, with a single difference: conversion of an electron from the material particle into the electromagnetic pulse and vice versa takes place within the range of temperatures and frequencies available for measuring at today level of science and technology 60000°K ≥Т (degrees) ≥1°K, while conversions in nucleons take place at temperatures beyond these limits: 6×107 °K ≤ Т (degrees) ≤1×10-3 °K.
Therefore, the author has accepted two assumptions:
1. 2n2/z is the number of quantum-states of an electron (groups of electrons);
2. There are conditions, under which an electron may be converted from the material particle into the electromagnetic pulse (which is explained by the so-called dualism).
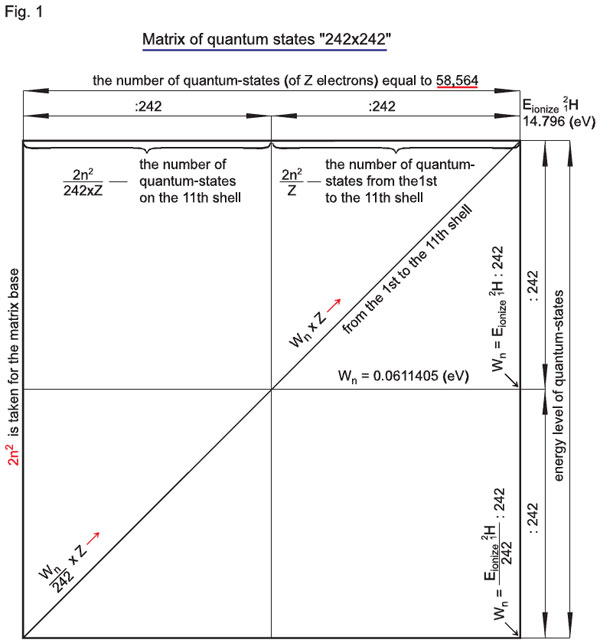
To follow the argument, let us assume that for the atom of hydrogen with charge z=1 ([H ) the number 2n2/z is equal to 2n2. Hence, the eleventh electron shell of the atom of hydrogen has 2n2 = 2 • 112 = 242 quantum-states of an electron. Yet, according to the interpretation given in courses of physics, this is just the number of electrons (quantum-states of electrons) on the eleventh shell. However, each of the eleven shells is also quantized by the energy potential in accordance with the formula En = E/n2.
Unlike helium (24Нe), the atom of hydrogen (11Н ) has just one electron on the first shell, and accordingly, there must be two quantum-states of an electron on the first shell of the atom of hydrogen. Yet in manuals (as well as in practical calculations) the formula En = E/n2 is used, which is true for helium (24He) in the «121 Ч 121» matrix (see below) but not true for hydrogen, as the calculation of quantum-states for helium is 2n2/z = 2n2/2 = n2, while for hydrogen it is 2n2/z = 2n2/1 = 2n2, therefore, the formula for hydrogen should look like En = E/2n2 — a hydrogen group 11Н — hydrogen;12Н —
deuterium; 13Н — tritium — in the «242 x 242» matrix (see below). In the author’s opinion, that is the reason (see the calculations below) behind the absence of dependence of λ and v (i.e. wave-length and radiation frequency) on temperature, at which λ • ν ≠ с (с = 3 • 108 m/sec).
So, from the first to the eleventh shells of the atom of hydrogen (11H), the number of energy quantum-states is En = E/2n2 = Е/2•112 = Е/2•121 = Е/242.
As a result, we have Е/242 = 242Wn, i.e. two hundred and forty two energy quantum-states of an electron from the first to the eleventh shells, and 2n2 = 242 quantum-states of an electron on the eleventh shell itself.
As the whole, we have 2n2 x 2n2 =242 Ч 242 = 58564 quantum-states of an electron for the atom of hydrogen (\H ).
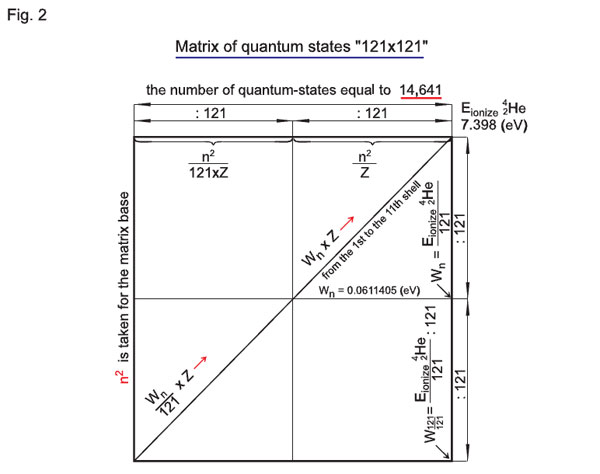
With the atom of helium (4He), this scheme will look like 2n1/z Ч 2n1/z = n2 x n2 = 121 Ч 121 = 14641 quantum-states for a group of electrons (group of two atoms).
Therefore, 2n1/z Ч 2n1/z is the number of quantum-states of electrons (group of electrons z) for any atom with charge z. Here, the first multiplier 2n2/z corresponds to quantum-states of the potential from the first to the eleventh shells, and the second multiplier 2n1/z corresponds to the quantum-state of an electron on the eleventh shell itself.
That is the approach to be taken for the basis for concordance of atoms of any elements from the periodic table, i.e. an effort to create the «single coordinates». Let us define the schemes for determination of quantum-states for hydrogen «242 x 242» and helium «121 x 121» as matrices (2n1/z x 2n1/z is a matrix).
These matrices of quantum-states of an electron (groups of electrons) are presented in the attached figures, where:
Fig. 1 presents the «242 x 242» matrix;
Fig. 2 presents the «121 x 121» matrix;
Fig. 3 presents the combined matrices (it can be seen from Fig.3 that the number of quantum-states of the «242 x 242» matrix is equal to 58564, which is four times bigger than the «size» or number of quantum-states of the «121 x 121» matrix; indeed, 58564 / 14641 = 4).

There is another acceptable method allowing to the atom electron shells to be «unfolded» and presented in the arc form. Here, the matrix will correspond not to the total number of quantum-states of electrons (groups of electrons), but a two-staged quantization (where the first stage covers from the first to the eleventh shells, while the second stage refers to the eleventh shell itself). Therefore, we can transform the matrix 2n1/z = n1/z + n1/z, which will substantially simplify understanding of ongoing processes while selecting the «single coordinates» for the calculation of parameters of atoms and coordination of their characteristics. The transformed matrix (this could be both «121 x 121» and «242 x 242») is presented in Fig. 4.
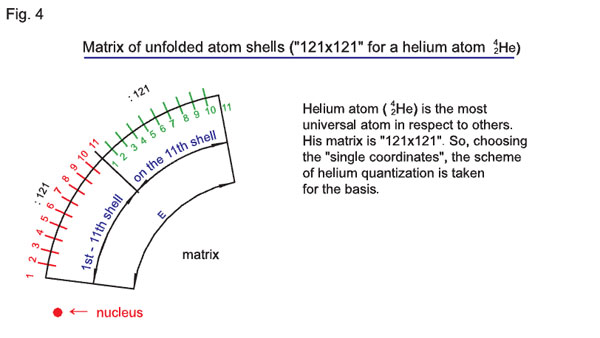
The most versatile atom in respect to all other atoms is the atom of helium (24He) with the «121 x 121» matrix.
Therefore, when selecting the «single coordinates» we take the scheme of helium quantizing for the basis.
As a result of the previous arguments, we came to the conclusion that 2n1/z may be presented not just as the number of electrons on the n-th shell but as (2n1/z)2, that is the number of quantum-states of an electron (groups of electrons) of the atom with charge z (see diagrams 1 and 2). 2n1/z = n1/z + n1/z refers to the unfolded atom shells (in the arc), where double quantization is taking place. The first stage of this double process covers from the first to the eleventh shells, the second stage takes place on the eleventh shell itself (see diagram 4).
Looking ahead, it has to be said that it is during the second quantization on the eleventh shell that electrons are converted from the material state (particle) into the energetic one (electromagnetic pulse). The eleventh shell of any atom is an indicator of resonant characteristics or quantum-states of the atom. It is on the eleventh shell that conditions are created for appearance of 2mс2, 4mс2, 8mс2, which are to be considered after calculation of the matrix parameters. The above-mentioned formulas and constants used in practice and will be applied for calculations.
First, let us determine the cause of inconsistency between dependencies of frequency ν and wave-length λ on temperature. This will be achieved through the formulations and interpretations of the Wien’s displacement law:
— frequency corresponding to the maximum value of spectral density of radiant exitance of a black body is proportional to its thermodynamic temperature;
— wave-length λmax corresponding to the maximum value of spectral density of radiant exitance of a black body is inversely proportional to its thermodynamic temperature:
U = b/Т, where b = 2.898 • 10-3 m • °K is the Wien’s constant (according to the author’s calculations, b = 2.898106 • 10-3 m • °K);
— λmax and νmax lie in different parts of the spectrum, and the values corresponding to them are not bound (emphasized by the author) by the relation λ = с/ν.
To eliminate the last inconsistency (or to explain it) further investigations into the type of the Kirchhoff’s function have been carried out.
The Rayleigh-Jeans’s law matched well the data of experiments in the range of low radiation frequencies only. For higher frequencies it was clearly wrong. Impossibility to find an expression of the Kirchhoff’ s function, which would match the experimental data throughout the whole frequency range (from 0 to ∞), was figuratively defined as «ultraviolet catastrophe». In 1910 M. Planck succeeded in finding the type of function dependency of frequency on temperature exactly corresponding to the experimental data, having introduced a notion of energy portions (quanta). The Planck’s constant is defined as proportionality coefficient h between energy Е and frequency ν: Е=hν.
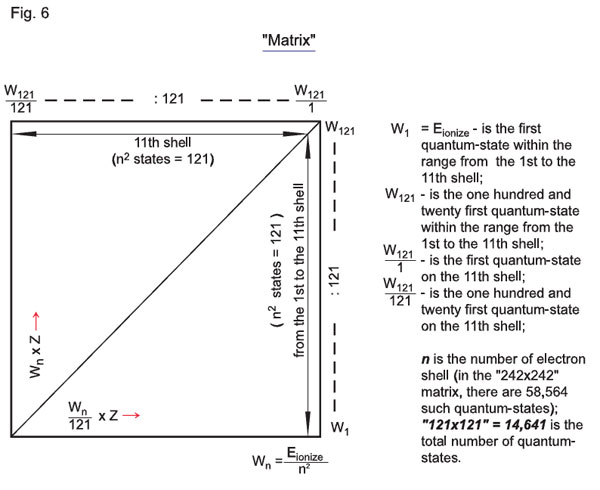
A simplified double-quantization matrix is presented in Fig.6, where at the first stage (from the first to the eleventh shells) quantization only covers Wn = E/n2, while at the eleventh shell itself it is the potential of the eleventh shell that is quantized (Wn/121):121; W1 = Еionization is the first quantum-state within the range from the first to the eleventh shell; W121 is the one hundred and twenty first quantum-state within the range from the first to the eleventh shell; W121/1 is the first quantum-state on the eleventh shell; W121/121 is the one hundred and twenty-first quantum-state on the eleventh shell; n is the number of an electron shell (in the «242 x 242» matrix, there are 58564 such quantum-states); «121 x 121» = 14641 is the total number of quantum-states.
However, from the Wien’s displacement law, the Wien’s constant b = 2.898 • 10-3 m • K, where this value exactly corresponds to the practical calculations.
The order of digits obtained in our calculations for hydrogen is 5. 3351 • 10-3 m, and the Wien’s constant b = 2.898 • 10-3 m • K, i.e. the difference between these two values makes it possible to suppose that the Wien’s constant refers to the atom of helium (24He), and not to the atom of hydrogen \H !
Having therefore passed to calculation of the atom of helium, let us first make the following step.
We are to recalculate the Wien’s constant b = 2.898 • 10 3 m • K into frequency λ • Т= 2,898 • 10 3 m • °K:
Both for helium (24He) and hydrogen (11Н ) the maximum possible value of νmax in the «single coordinates» (in the «121 x 121» matrix) may be equal to νmax = R / (Т • n (quantum-states)), where R = 3.2931193 • 1015 sec-1 per one quantum-state of temperature divided by one quantum-state of temperature. Then one quantum-state of temperature for helium will be equal to:
R 3.2931193-1015 3.2931193-1015
Therefore, for the atom of helium (24 He) each quantum-state (of two electrons) is assigned with 2.1728 K, whereas for hydrogen each quantum-state is assigned with 1.
Let us sum up the obtained results:
a) value of displacement for hydrogen λ11H = 5.3351 • 10-3 m;
value of the Wien’s displacement for helium λ24He = 2.898 • 10-3 m • °K;
b) frequency of quantum-states for hydrogen М11H = 562.311 • 108Hz;
frequency of quantum-states for helium М24He = 1035.19•108Hz/°K;
c) value of one quantum-state for hydrogen is 1;
value of one quantum-state for helium is 2.1728 °K.
Having compared units of measurement for hydrogen and helium and having taken into account that the order of digits is commensurated, we may assume that these units both for hydrogen and helium may be referred to the Kelvin degree.
Then, for one quantum-state for hydrogen (11H ), we obtain the following const:
HI = 2.32549 • 10-4 eV/°K; М = 562.31119 • 108 Hz /°K;
One quantum-state = 1°K.
Having taken into account that one quantum-state for the atom of hydrogen is equal to 1°K, and the total number of quantum-states for hydrogen (11H ) is 58564, we conclude that the maximum temperature of the atom of hydrogen corresponding to the temperature of ionization will be equal to 1°K • 58564 = 58564°K or
Тionization L ^ = 58564 K.
For helium (24Н e) one quantum-state is equal to 2.1728°K, and the total number of quantum-states is 14641. Correspondingly, the temperature of ionization will be equal to 2.1728°K • 14641 = 31812°K or
Тionization 2 He = 31812 K.
It is to be emphasized that the last electron pair of helium (the last quantum-state) has the potential of 2.1728°K, accordingly, there are no electrons below this temperature (they are converted from the state of the material particle into the energetic state), that is the reason of helium II being superfluid. What is going on here is defined as a helium phase modification: helium I is transformed into helium II.
Based on the fact that, according to the above calculations, the last quantum-state, at which helium still has electrons, is registered at the temperature of 2.1728°K, while below this temperature there are no electrons as such, and this temperature level matching the practically obtained temperature level, at which (below 2.1728°K) helium becomes superfluid, we may conclude that our approach to interrelation of hydrogen and helium is true.
Moreover, this explanation of superfluidity of helium (2 He) is the most objective and clear from the point of view of
common sense: in the absence of electrons, the atom of helium becomes just a nucleus (in its material sense) and correspondingly, it is easy to calculate how much smaller the diameter of this material nucleus has become, compared to the diameter of the atom of helium, when there are electrons even on the first shell, no to mention the third, fifth, etc. shells! Besides (which is also very important), we have obtained an explanation of why the Wien’s displacement constant b = 2.898 • 10-3 m • °K, having good practical results, did not match the function of frequency on temperature. The reason turned out to lie in the fact that λ of displacement was practically determined for helium (11Нe), while ν of displacement was determined for hydrogen (11Н ).
Taking into account that the helium nucleus charge is twice as large as the hydrogen nucleus charge, Еionization 2 4He should also become twice as small, yet such decrease factor only equal to 1.8409 times. It is related to the fact that the atom of hydrogen has just one proton and no neutrons, while the nucleus of the atom of helium has two protons and two neutrons.
Let us apply this fact for establishing interrelations between the atoms of hydrogen and its isotopes: deuterium 12Н and tritium 13H , as well as relation between helium I He and its isotope 23Н e.
Knowing Еionization 23Нe , we may proceed to a nucleus with charge z = 1 (i.e. hydrogen) and make a clear assumption that for the isotope of hydrogen — deuterium — 11Н , which has one proton and one neutron, the potential Eionization f# will make two potentials Еionization 2 #e> as these atoms are absolutely symmetrical in terms of composition of their nuclei: deuterium 12H has one proton and one neutron, helium 24Нe has two protons and two neutrons. Accordingly, Eionization \H = Еionization 24He • 2 = 14.796 eV or Еionization12H = 14.796 eV.