As was mentioned above, hydrogen is not «symmetrical» to helium corresponding to the «121 x 121» matrix. Therefore, the basis of the hydrogen group «242 x 242» matrix will be constituted by hydrogen isotope 11Н , its parameters being absolutely symmetrical to the parameters of helium, and accordingly, there will be an absolute interrelation between helium 24He matrix («121 x 121») and deuterium 12Н matrix («242 x 242»).
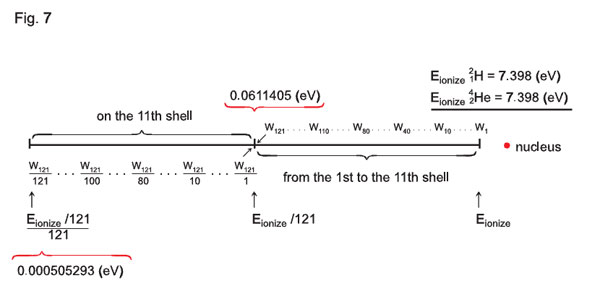
Еionization12Н of deuterium is equal to 14.796 eV. Hence, the last quantum-state from the first to the eleventh shell will have the potential equal to 14.796 eV / 242 = 0.0611405 eV.
Еionization 24Нe °f helium is equal to 7.398 eV. Hence, the last quantum-state the first to the eleventh shell will have the potential equal to 7.398 eV /121 = 0.0611405 eV.
Let us analyze the formula, which makes it possible to determine the energy of an electron in the electrostatic field of the nucleus for the «121 x 121» matrix:
It may be noticed that with z = n, z1/n1 = 1. Accordingly, Wn will be always equal to the same value of Еionization of the basic element of the matrix or correspond to the potential of the first quantum state in the base matrix divided on z (nucleus charge):
Z=1: In the «242 x 242» matrix — to deuterium 12Н (Еionization12H= 14.796 eV);
Z=2: In the «121 x 121» matrix — to helium 24Нe (Еionization 24He= 7.398 eV).
It is known that z is the nucleus charge. As we consider relation between the atoms electron shells, n is the number of an electron shell, while the potential present on the last electron shell may be found in the following way: Wn = Еionization/n2, i.e. the smallest value of the potential on the eleventh shell is 121 times smaller than Еionization (in the «242 x 242» matrix there are 2n2 quantum-states of an electron on the eleventh shell, or 2 • 112 = 242 quantum-states. In the «121 x 121» matrix there will be 121 such quantum-states, and further on we are to consider the formula for the «121 x 121» matrix). Then we conclude that 1/121 Еionization on the eleventh shell is also distributed among 121 quantum-states.
Wn = — z RH , n2 will not only mean «the squared number of n
electron shell» (yet, it is also quantized the energy state, though for shells), but «the squared state» (of electron), i.e. 121 quantum-states, we are to obtain very interesting results, which by no means conflict with the theoretical arguments.
Thus, based on these considerations, we may present the electron shell as a matrix, where (or on which) the energy is quantized not only by shells, but by quantum-states of an electron (groups of electrons), see Fig.7.
We may suppose that the periodic table is not completed, and it may well have element 121 (which is characterized by antigravity properties, when the nucleus acquires a proton-neutron equivalent, a new state — see below).
Then, with z = 121 and n = 121 (and that is the potential of Еionization/121 or the energy potential of the eleventh electron shell), we may observe that the eleventh shell of the atom with charge z = 121 will take the place of the first shell of the basic atom in the «121 Ч 121» matrix, i.e. the first shell of the atom of helium \ He, and the last sublevel of the eleventh shell of the atom with charge z = 121 will take place of Wn = Еionization/121, i.e. the eleventh shell of the atom of helium 24He (see Fig. 8).
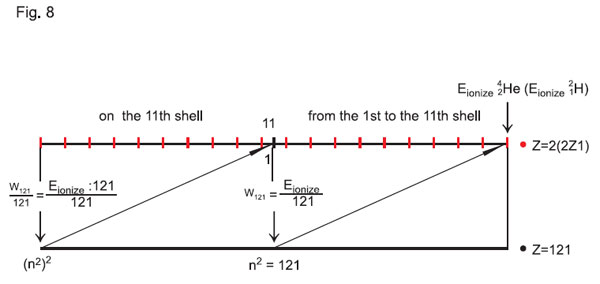
It has to be emphasized that the schematic presentation corresponds to the relation between electron shells of atoms with charge z and the atom of hydrogen, though the text says about relation between the shells in the «121 Ч 121» matrix and the atom of helium \ He. This is not an error of having converted hydrogen 11H from the «242 Ч 242» matrix into the
«121 Ч 121» matrix (see below), this was done to create a kind of «symmetry» between the atoms of all elements of the periodic table, we have transformed hydrogen in such a way that in the «121 Ч 121» matrix it gets converted from Н1 into Н2 (or \H), and its electron shells in the «121 Ч 121» matrix
totally coincide with arrangement of the helium 24He electron shells (relative to the nucleus). That is the «price» we had to pay for creation of the single system of calculations for atoms, yet we imply that the constants (const), which we earlier obtained for hydrogen, are universal and may be used both in the «121 Ч 121» matrix, and in the «242 Ч 242» matrix:
HI = 2.32549 • 10-4 eV/degree;
M = 562.311•108 Hz/degree.
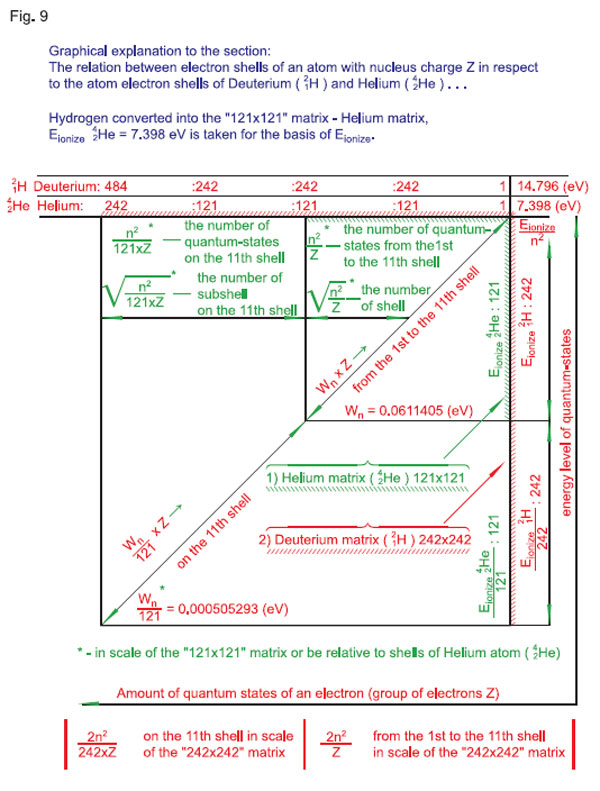
Therefore, in the «121 x 121» matrix, the first and the second quantum-states of an electron of the atom of hydrogen are still located on the first electron shell, just as the first quantum-state of the atom of helium is located on the first electron shell. And as locations of the first electron shells for helium and hydrogen are matched through scaling, schematic presentation of variation (displacement) of similar shells of atoms with charge z relative to the shells of the atom of hydrogen is also true and clear for understanding.
Therefore, we may conclude that the matrix (see Fig. 9) where with hydrogen (hydrogen isotope deuterium 12Н) converted into the «121 x 121» matrix (helium matrix), Еionization 24Н e= 7.398 eV is taken for the basis of Еionization) corresponds to the quantization of:
Let us return to analysis of Fig. 8. The nucleus with charge z = 121 with a totally filled electron shell is in a state, which corresponds to the quantum-state of the nucleus. Here, we may observe both antigravity properties of the atom with charge z = 121, and transition of the nucleus itself into another energy state: from the proton-neutron state into a new type (new state). I.e., a nucleus, as well as an electron, should have such a state, in which one energy type becomes converted into another energy type (another state).
The nucleus with charge z = 121 most likely belongs to the atom, any further change of which is related to change in the nucleus, i.e. quantization of the nucleus will necessarily take place.
Let us consider more in detail quantization with the potential equal to 0.0611405 eV. We are to designate this quantum-state as Wquant = 0.0611405 eV and express this potential through Ш = 2.232549 • 10-4 eV/degree. We shall have:
(We take Kelvin degrees for the basic unit of temperature, as all the calculations are only made in Kelvin degrees).
As we convert all the atoms into the «single coordinates»:
for helium 4He — in the «121 Ч 121» matrix;
for deuterium \H — in the «242 Ч 242» matrix,
then Wquant and Тquant will be taken as the basis for calculation of displacement of the electron shells for other atoms as well. Here, it has to be taken into account that the quantum-state of the nucleus with charge z will be z times as large as Wquant and Тquant.
Let us apply this method for calculation of any element from the periodic table, taking out an element, which is the most common and widely used in practice, to compare our theoretical calculations to practical results.
The characteristic most cited in manuals is that of mercury, its nucleus having charge z = 80.
Wquant (z80) = 0.0611405 eV • 80 = 4.891 eV.
In the 121 Ч 121 coordinates (for the atom of helium 24He) mercury will have the number of quantized states equal to:
121 / 80 = 1.5125, which will be equal to potential being:
4.891 eV • 1.5125 = 7.39763, i.e. close to the energy of helium ionization.
However in the «242 Ч 242» coordinates (of hydrogen, and more precisely of the hydrogen isotope deuterium\H) the number of quantum-states for mercury will be equal to: 242 / 80 = 3.025, i.e. there will be three whole states for 4.891 eV. Hence, the resonance (quantized) states for mercury in the «242 x 242» coordinates will be:
The first one-4.891 eV;
The second one — 4.891 eV • 2 = 9.782 eV;
The third one — 4.891 eV • 3 = 14.673 eV.
The total result with the quantization factor being 3.025, we have:
4.891 eV • 3.025 = 14.795 … eV, or the energy of ionization of the atom of hydrogen isotope (deuterium 21H).
Next, we are to apply the same technique to determine through Тquant the quantization temperature, to be further used to find the resonant frequency and its corresponding to wave¬length λ through const М = 562.311 • 108 Hz/0K.
We are now to check the obtained results to those already known from manuals with reference to practical data.
Potentials of the resonant values of the energy levels show exact matching: 4.891 eV; 9.782 eV; 14.673 eV.
The wave-length for the first quantum-state of mercury with 4.891 eV:
X (z80) = 2536.535 A, whereas in books and manuals on physics this value is 2536.5 A.
Thus, the results of calculations coincide, which means that the conclusions made about displacement of electron shells of atoms with charge z with respect to the atom of helium 24He(the «121 Ч 121» matrix) and the atom of hydrogen
isotope — deuterium \H (the «242 Ч 242» matrix), are true.
The continuous spectrum corresponding to quantized states of frequencies is determined for the «121 Ч 121» matrix: