In a carbon-hydrogen mixture, the prevailing compounds are «С — С» and «С — Н». To change chemical-physical properties of oil, the latter should be influenced upon. As the rule, such influence is produced by means of heating a hydrocarbon mixture (oil) and its fractionation.
But what is the temperature range, in which it would be possible to obtain the maximum effect? And what else should be done to raise the efficiency of acting upon oil, to increase the output of light oil products (diesel, kerosene, benzene fractions).
As it is known from practice, the principal reserve is acting upon heavy molecules within the range of temperatures from 350°С to 500°С and higher (to make these molecules disintegrate into lighter ones, which brings about increase of the output of light oil products). In practice, oil-processing enterprises use an expensive technique (multiple vacuum distillation).
However, there is a more efficient method, which does not require expensive installations and allows obtaining up to 70% light oil products (of the supplied amount of oil) with just one atmospheric column used.
The essence of this method lies in the knowledge of behavior of hydrogen atoms at resonant frequencies (temperatures), when (with minimum energy input «from outside») the «С — Н» bonds are broken. As a result, the number of light molecules is increased, which gives an increase of the output of light oil products.
So, to affect heavy molecules in the temperature range above 350°С, we use the resonant characteristic of hydrogen.
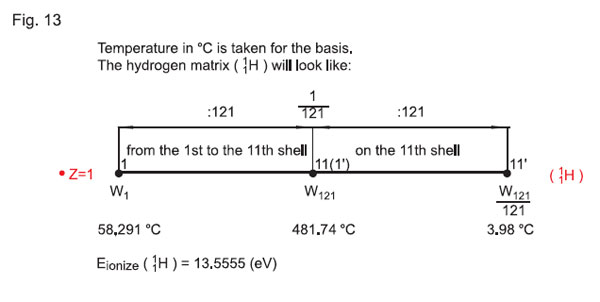
Taking hydrogen 11Н for the basis, in the «121 x 121» matrix we shall have:
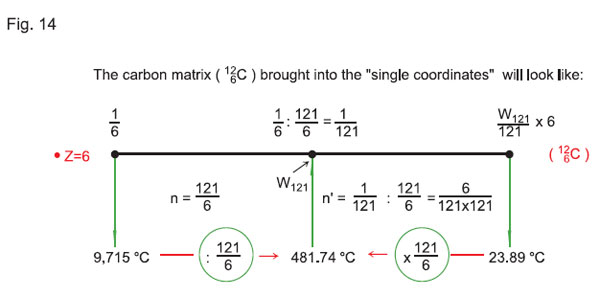
The minimum temperature (per one quantum-state) will correspondingly be equal to 4°K or 3.98°С. Let us take temperature in °С for the basis. The matrix of hydrogen 11 will look as presented in Fig. 13, while the matrix of carbon 126C reduced to the single coordinates is presented in Fig. 14.
Therefore, applying the rule of displacement of the electron shells (see above), we can see that on the first electron shell of the atom of carbon (in the scale of the «121 x 121» matrix on the sixth quantum-state for hydrogen 11Н) the temperature is 58291°С / z = 58291 / 6 = 9715°С.
These explanations are sufficient for understanding. Further calculations are made in a relatively simple way.
The basis of the calculation may be either energy potential Е, to be further converted in temperature through the formula Е = 2.32549 • 10-4 • Т° or immediately the temperature itself (Тionization).
Let us adopt for calculation the simplest option, i.e. calculation through Тionization 11Н= 58564°K.
Let us calculate resonant frequencies and temperature range of the «С — Н» compound. As two atoms 126С and 11Н are interconnected, they affect upon each other, therefore the resonant temperature will have the range from Tmin to Tmax.
Let us begin our calculation from carbon 126С, as it will define the main characteristics (of the bond) due to the fact its charge and weight are six times as big as those of hydrogen 1Н, and, correspondingly, it will define the maximum constituent of equilibrium radiation.
As the temperature of ionization of hydrogen (Тionization11Н) is equal to 58564°K 13.619 eV / (2.32549 • 10-4 eV/degree), all other atoms with charge z (sum of oscillators) will have the temperature of equilibrium radiation on the first own electron shell in the «121 Ч 121» matrix z times lower.
For carbon126С, z = 6, therefore the temperature on the first shell of the atom of carbon (in the scale of the matrix of hydrogen) will be six times lower than Tionization 11Н.
To proceed from °K to °С, let us apply the approach A ionization ( 0С) = Т ionization ( 0K) – 273 (see above), then Т ionization 11Н (°С) = 58564°K — 273 = 58291°С.
As 126С has charge z = 6, Тionization (°С) of the atom of carbon, with n = 1 (when the atom is unexcited) will be equal to:
Тionization 126С (°С) = 58291°С / 6 = 9715°С.
According to the theory of the state of thermal equilibrium and equilibrium thermal radiation, if a charge (number of oscillators) increases with a factor equal to К, based on the formula Е = h • ν, frequency ν should also increase with a factor of К (as h — Planck’s constant has a fixed value).
At the same time, ν = R / n2, where R is the Rydberg’s constant, which is also fixed. Correspondingly, to make frequency increase with a factor of К (compared to hydrogen \H, К = z times), the number of n2 (the number of an electron shell) should become К or z as low (that is another variant confirming that the adopted approach to quantization (displacement) of electron shells is true).
Therefore, for the atom of carbon126С, the resonant frequency corresponding to the resonant frequency of the atom of hydrogen in the scale of the «121 Ч 121» matrix will be on the shell (in the quantum-state) n2 / 2 = 121 / 6 = 20.166… . We have taken n2 = 121, i.e. the resonant frequency of the atom of hydrogen will be found on the eleventh shell, or on the first sublevel of the eleventh shell (W121 = Еionization /121).
As we know, n2 / z is the number of quantum-states (20.166… • 481.74°С = 9715°C), while the square root of n2 / z is the number of shell (from the first shell to the eleventh shell). Hence, for the atom of carbon 126С n will be equal to:
n = n2z =20.166… =4.49.
Let us calculate temperatures for n = 4 and n = 5 of the atom of carbon:
Т4 (°С) for 126С= 9715 /n2 = 9715 /16 = 607.2°С;
Т5 (°С) for 126С= 9715 /n2 = 9715 /25 = 388.6°С. Here, the resonant temperature is calculated as follows:
Тresonance 12С= 9715 / 20.166… = 481.74°С.
However, this resonant temperature (481.74°С) does not fall to an integer number of the electron shell (4.49), therefore, it will only appear (be enabled) due to the external factors, rather than the atom of carbon itself. This will be the very case, when we shall excite the С — Н bond through the resonant constituent of hydrogen, acting upon it (481.74°С). This Тresonance is used in the invention to achieve an increase of the output of light oil products.
So, what the result can we get with n = 4 and n = 5 ?
Т4 = 607.2°С; Т5 = 388.6 °С.
However, we have to determine the range of temperatures for the С — Н bond; knowing that the nucleus charge and weight of hydrogen \H are six times smaller than those of carbon 126С, let us determine the percentage ratio between these numbers from the relation z \HI z 126С= 1/6.
Since we have two oscillators taken together, temperature Т, which is common for the equilibrium state and equilibrium radiation for 126С will be changed (decreased) in
the С — Н bond by (1 / 6) • 100% = 16.66% and the following temperature range will be formed:
1. Т4max=607.2°C;
T4min= 607.2°C — 16.6% = 506°C;
2. Т5max = 388.6 C;
T5min= 388.6°C — 16.6% = 323.9°C.
It can be seen from these calculations, that they are based on the above-mentioned materials.
To make the reasoning more illustrative, and to compare the obtained results to the practical ones, Fig. 15 and Fig. 16 are supplemented presenting an oil diagram.

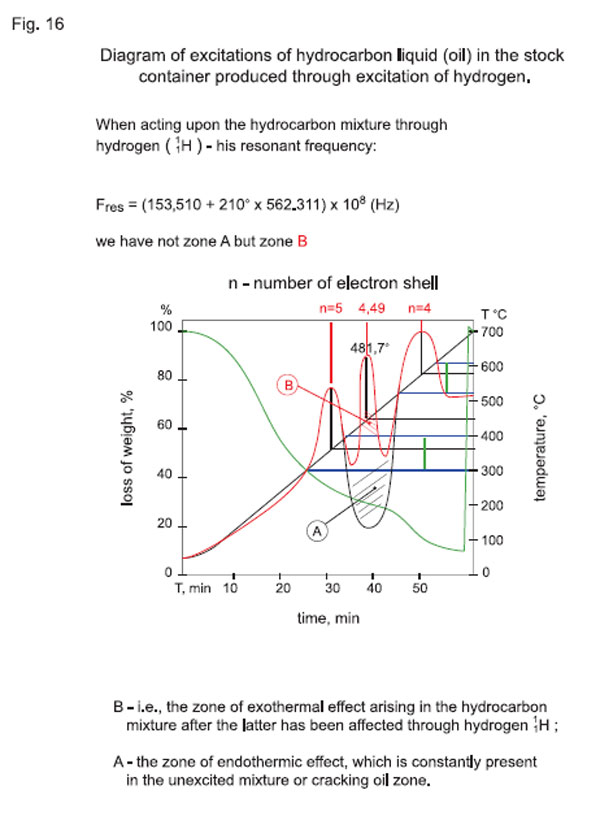
When acting upon the hydrocarbon mixture through 11Н,
we have not zone А (see Fig. 15), i.e. the zone of endothermic effect, which is constantly present in the unexcited mixture or cracking zone, but zone B (see Fig. 16), i.e. the zone of exothermal effect arising in the hydrocarbon mixture after the latter has been affected through hydrogen 11Н, made in the laboratory conditions with an instrument, and showing the relation between the oil weight and its temperature. Resonant temperature ranges of oil (which match almost exactly the above-obtained calculated results) can be clearly seen in the diagram.
Deviations are common, but they are not very significant and are characterized by oil quality (its purity), i.e. content of sulfur, paraffin, asphalt-tar substances, etc.
A specimen under investigation was heated up to 700 °С, therefore the calculated temperatures are presented in the graph in an illustrative and complete way (throughout the whole temperature range).