In everyday life, we constantly use the temperature scale in Celsius degrees. The ratio between temperatures in Celsius and Kelvin degrees is well-known: the difference between the values of the same measurement of state of a body in °K and °С will be equal to 273, i.e. 273°K = 0°С, 0°K = -273°С, etc. Relation between these temperatures (with the difference equal to 273) on the linear scale is presented in Fig. 10. As the Celsius scale is the most widely used, let us take the temperature presentation in Celsius degrees for the basis. The only (yet, very important) addition for further reasoning presented in Fig. 10 is the relation of temperatures in °С and °K through const Ш = 2.32549 • 10-4 eV/degree to the energy potential, namely to Еionization11Н =13.619 eV.
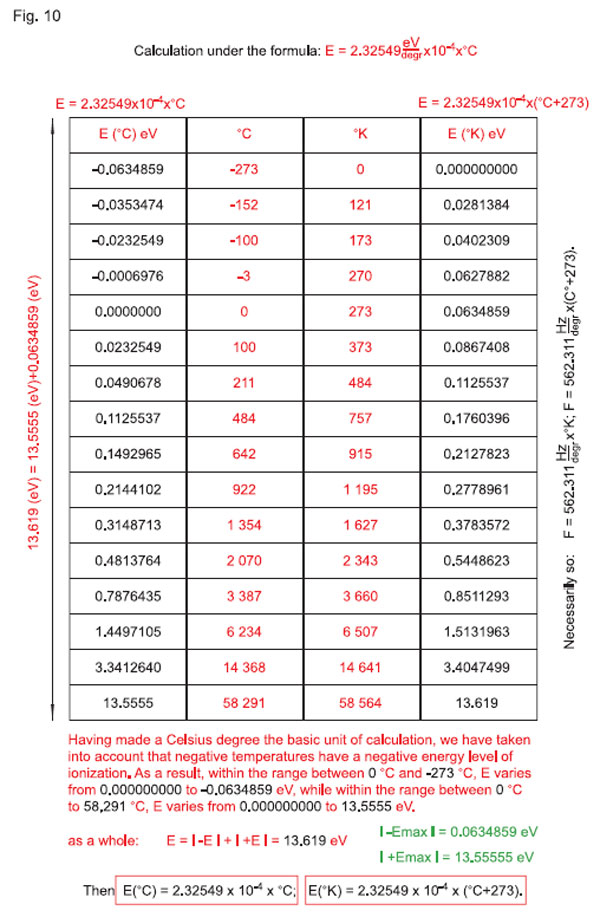
Having made a Celsius degree the basic unit of calculation, we have taken into account that negative temperatures have a negative energy level of ionization. As a result, within the range between 0 °С and -273 °С, Е varies from 0.0000000 to -0.0634859 eV, while within the range between 0 °С to 58291 °С (58564-273=58291), Е varies from 0.0000000 to 13.5555 eV. Note that | +Еmax | = 13.55555 eV, | -Emin | = 0.0634859 eV, as a result, Е = | -Е | + | +Е | = 13.619 eV. Then
E (°С) = 2.32549 • 10-4 • °С; Е (°K) = 2.32549 • 10-4 • (°С + 273).
If we are to introduce the single coordinates for the temperature range as well (in Celsius degrees), conversion of calculation results from Kelvin into Celsius degrees will be fulfilled according to the following relation:
°С = °С;
°K = 273 + °С,
i.e., the values of Kelvin degrees are converted into Celsius degrees.
Then calculation of the potential corresponding to a specific temperature range (both in Celsius degrees and in Kelvin degrees through °С) will be performed by means of the following formulas:
E (°С) = 2.32549 • 10-4 • °С;
E (°K) = 2.32549 • 10-4 • (°С + 273).
It is crucial to pay attention to the fact that using these formulas we can determine the energy potential for negative temperatures in Celsius degrees, however, we cannot determine frequency through a negative potential, as frequency cannot be negative in principle: it is either present (positive) or absent (equal to zero). That is why it is very important to remember that, when proceeding to calculation of frequencies through const М = 562.311 Hz/degree, it is necessary to apply the formula in the scale of Kelvin degrees or (273 + °С), i.e. the correct calculation of frequencies may only be obtained with the following formulas:
f= 562.311 • 108 • Т(°K), Hz;
f = 562.311 • 108 • (273 + °С), Hz.
It is due to the fact that quantization of frequencies in the «121 Ч 121» matrix (in particular, for hydrogen) is only performed within the positive range of the potential Е and temperatures Т.
Having converted Еionization 11Н from the «Kelvin
degree» range into °С, we shall have 13.619 eV — (273 • 2.23549 • 10-4 eV). Then 11Н(°С) = 13.5555 eV.
Having converted Тionization 11Н from the «Kelvin degree» range into °С, we shall have 58564°K — 273=58291°С. Then
Тionization 11 { C) = 58291 C.
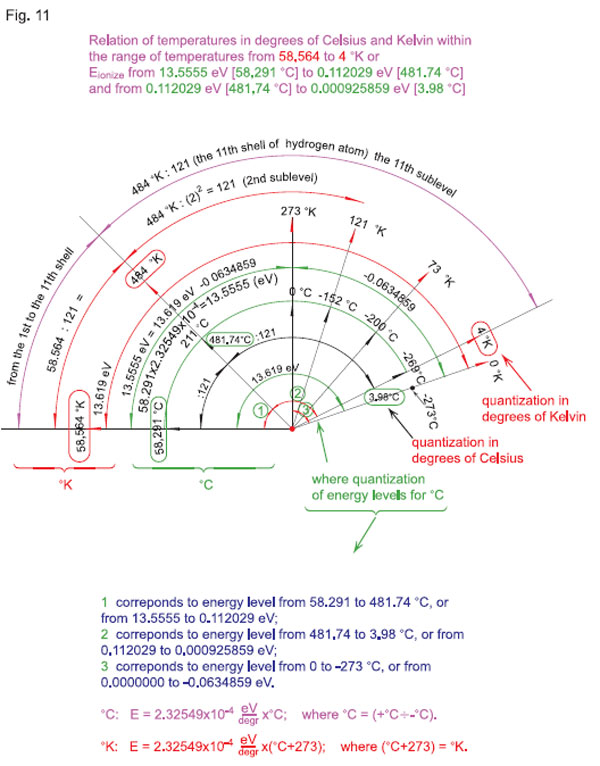
Next (see Fig. 11), where quantization of the energy levels for °С is conventionally presented in the following way:
1. corresponds to the energy level from 58291°С to 481.74°С or from 13.5555 eV to 0.112029 eV;
2. corresponds to the energy level from 481.74°С to 3.98°С or from 0.112029 eV to 0.000925859 eV;
3. corresponds to the energy level from 0 to -273°С or from 0.0000000 eV to -0.0634859 eV,
let us make a parallel of quantization in the same scale (in the «121 Ч 121» matrix) of temperatures in °K and °С, and note that between the linear scale (dependency) of these temperatures and the scale of quantum-states on electron shells of the atom (in particular, that of hydrogen 11Н) there is a very significant difference, which has never before been taken into account in theory or in practice (see Fig. 12).
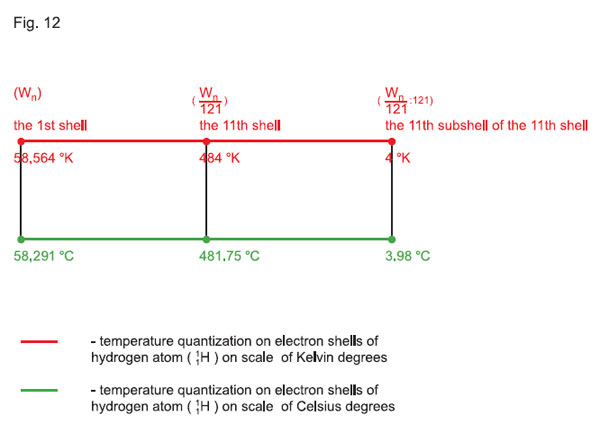
Therefore, from the above-mentioned values of temperatures, as well as from (Figs. 11 and 12) we can make sure that during quantization by quantum-states of the atom (of hydrogen), negative temperatures (°С) are not quantized. That is why, doing research in laboratory conditions, with the °С scale applied, it is necessary (determining frequencies) to introduce an offset for recalculation of temperatures in Kelvin degrees: °K = 273 + °С, or it is necessary to add an offset to the obtained value of frequency: (562.311 • 108 • 273) Hz, i.e., to add the above value. Such a correction will give the actual (practical) value of frequency.
On the eleventh sub shell of the eleventh shell of the atom of hydrogen, we can see the value of the first quantum-state in the «121 x 121» matrix, equal to 4°K.
Thus we can confirm once again that our arguments are true in what concerns the absence of quantization on the shells of atoms of negative temperatures and necessity (while measuring temperatures in °С) to introduce an offset for determination of the actual frequency at:
(562.311 • 108 • 273) Hz = 153.511 • 108 Hz.
Thus, to determine the energy potential through the value of temperature, we have the following formulas:
in Celsius degrees: Е = 2.32549 • 10-4 • °С, eV, 0≤°С≤0;
in Kelvin degrees: Е = 2.32549 • 10-4 • °K, eV, 0≤°K.
To determine frequency through the value of temperature, we have the following formulas:
in Celsius degrees: F = 562.311 • 108 • (273 + °С), Hz, 0<°C<0;
in Kelvin degrees: F = 562.311 • 108 • °K, Hz, 0≤K.
The constant values:
HI = 2.32549 • 10-4 eV/degree, М = 562.311 • 108 Hz/degree
are the universal const, as they have the same values both for 1 °C, and for 1°K. That is why, their more correct unit would be "per one degree", regardless of which degree is used that of Celsius or Kelvin.